| Background > Water column profiles
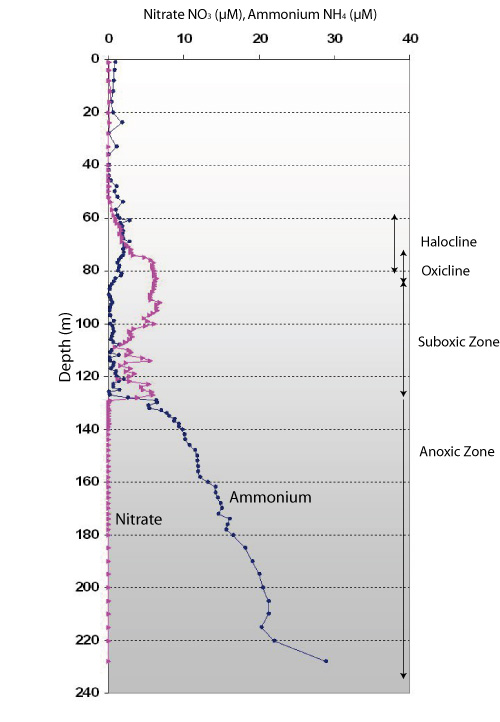
Nitrate is depleted in the surface water, partly regenerated in the suboxic zone, and completely removed in the anoxic zone
Nutrient concentrations in sea water are always the result of the two processes of production and consumption. This is called "turnover". Only if more is produced than consumed, nutrients can accumulate. This differs considerably during the course of the year, and in different depth zones.
Nitrogen is the most important nutrient for phytoplankton growth in the Baltic proper. It is available in three forms: nitrate, nitrite and ammonium (nitrite is usually only produced as a intermediate step in a process called "nitrification" and only available in very low amounts, to be shown in the next profile).
Nitrate (pink symbols) as the most important available nitrogen species, is completely exhausted in the sunlit surface water. This is usually the case in the Baltic proper during most of the summer, after spring blooms have consumed all nitrate in the euphotic zone, i.e. the sunlit surface water where photosynthesis can occur. During the summer period, phytoplankton growth mostly relies on ammonium, which is readily recycled within the surface layer by hetrotrophic organisms. Ammonum (blue symbols) is present in the surface layer only in small amounts, but it is constantly turned over by autotrophic and heterotrophic organisms in the surface layer.
Interesting transformations occur in the sub- and anoxic zones: we can see that nitrate values increase in the layer below 55m, but then completely vanish below 130 m. The nitrate producing process in this layer is called "nitrification". Certain bacteria are responsible for the transformation of ammonium via nitrite (see next profile) to nitrate. This process requires oxygen, and thus is active in the oxic and suboxic layers, but not in the anoxic deep layer. The bacteria accumulate in the halocline; further up, their concentrations are too low to accumulate detectable nitrate.
As the anoxic zone begins, all nitate is gone. This is due to a microbial process called "denitrification". Here, specific bacteria use all the nitrate and nitrite for their energy metabolism, therby stripping the anoxic water from these nitrogen species, and producing molecular nitrogen N2. This process is crucial for the nutrient budget of the Baltic Sea, because it effectively removes nitrogen from the system, not only transferring it into other usable forms. With the exception of the notorious filamentous cyanobacteria, the end product of denitrification N2 cannot be used by phytoplankton.
Respiration processes by protists and crustacea producing ammonium (blue symbols) prevail in the halocline and oxicline, but not below. There is not enough oxygen of these organisms below the oxicline. Still, the ammonium concentration rises dramatically in the anoxic zone: this is not due to respiration, but to anaerobic decomposition of organic matter.
Go on to the nitrite profile...
|

